|
|
Sunday Workshops
Two sessions of 2 workshops will be held during the afternoon of Sunday, October 9. Please register during the registration process. Each workshop is available at a fee of 85 euro.
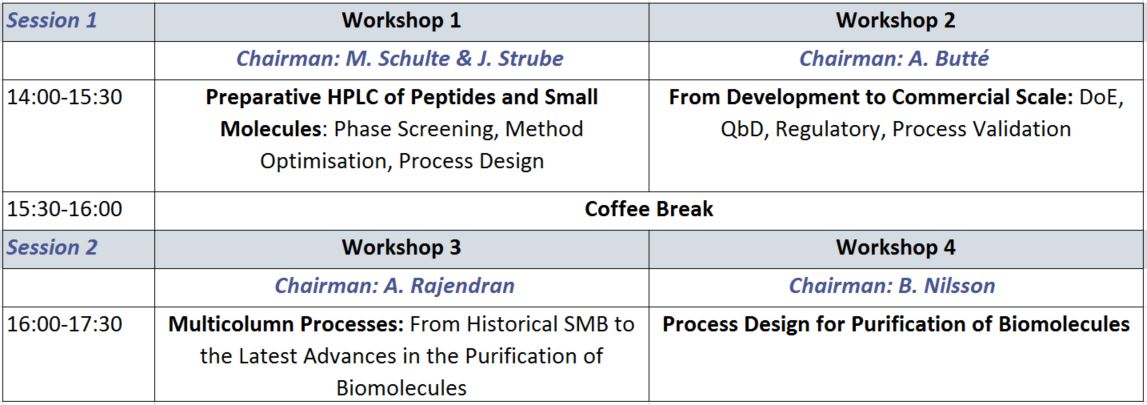
Session 1 | 14:00-15:30
Workshop 1: Preparative HPLC of Peptides and Small Molecules: Phase Screening, Method Optimisation, Process Design
Abstract
Where to start in preparative HPLC when a peptide or a complex active pharmaceutical ingredient has to be isolated in large scale? For many of the substances to be purified by preparative HPLC a certain method development and process design is needed. Either a generic method has to be developed which is able to separate a large number of substances following a certain protocol or – for production purposes – a specialized, highly optimized and economic production route has to be developed. This workshop will try to help developing a good, robust and efficient preparative HPLC method.
Using the example of the polishing step for the final purification of insulin you will learn about the importance of stationary and mobile phase selection. The workshop will demonstrate how methods developed at analytical column dimensions can be used to optimize a preparative method in different chromatographic regimes. Experiments to determine the physical properties needed for model based process design will be explained. Finally interactive simulation tutorials will be offered on batch and multi-column chromatography to optimize the process parameters.
Keywords
Preparative HPLC, phase screening, method development, stationary and mobile phase selection, process design, simulation tutorial
Lecturers
Michael Schulte is Director of Chromatography R&D at Merck KGaA. He did is Ph.D. work in the group of Professor Blaschke at the University of Münster, developing new chiral stationary phases. Since joining Merck in 1995 he has more than 20 years of experience in stationary phase design and process development in preparative chromatography.
Jochen Strube, is Univ.-Prof. Dr.-Ing. habil. at Clausthal University of Technology, Institute for Separation and Process Technology and director of that Institute since 6 years. Before that, Jochen has worked 7 years at Bayer Technology Services AG/Leverkusen in advance and education as chemical engineer at University of Dortmund with Diploma, Dr.-Ing and Habilitation.
|
Workshop 2: From Development to Commercial Scale: DoE, QbD, Regulatory, Process Validation
Abstract
In this workshop, the complex path that is connecting the choice of the appropriate purification method to the start of a production campaign is discussed. The choice of the purification process will be challenged from different perspectives, which are often enter in conflict with the classical tools to evaluate the performance of a purification, like yield, throughput. All side aspects of a purification process will be discussed, starting from tank volumes, sampling, pooling, storage conditions, hold times, waste treatment, etc., to finish with how the process fits to the rest of process and, most importantly, to the final isolation step. An important part of the discussion will focus on the regulatory aspects connected to the development and validation of the process, like the definition of the critical process parameters, and on how concepts like Quality by Design (QbD) can help correctly framing the scale up process and dealing with the risks associated to it. Basic concepts of Design of Experiments (DoE) and Multi-Variate Data Analysis (MVDA) will be also introduced.
Lecturer
Alessandro Butté received his Ph.D. in Chemical Engineering in 2000 from ETH Zurich. After a two-year post-doc at the Georgia Institute of Technology, he join the group of Prof. Morbidelli at ETH Zurich and completed his habilitation in 2008. During this period, his research activities focused on polymer engineering, production of nano-materials for protein purification (monoliths by reactive-gelation) and chromatography purifications of peptides, proteins and Mabs. In 2008, he joined Lonza as responsible for downstream activities in the sectors small molecules and peptides and as project manager. He was also involved in the pilot program to introduce Quality by Design into R&D. In 2013, he joined back ETH as senior researcher to further develop the latter concepts. He is author of more than 50 papers on international peer reviewed journals and several book chapters.
|
Session 2 | 16:00-17:30
Workshop 3: Multicolumn Processes: From Historical SMB to the Latest Advances in the Purification of Biomolecules
Abstract
Multi-column (MCC) processes have become the workhorse of chromatographic separations. They are able to overcome the limitations of low throughputs and excessive solvent consumption that characterize conventional single-column processes. Despite the higher complexity as compared to single column processes, the design and operation of a MCC separations is in fact rather simple. The workshop will trace the developments in MCC starting from the classical SMB mainly used for small molecule purification and slowly progress to more recent innovations related to large-molecule purifications. The workshop will be held in three parts.
The first part aims at providing the fundamentals of the SMB technology. We will cover the basics of SMB technology: modeling, design, and optimization of SMBs; engineering issues associated to the industrial use of SMBs.
The second part covers recent advancements in multi-column chromatography. The increasing use of the SMB as a multipurpose unit in the pharmaceutical and fine chemistry industries has led to the development of novel cyclic operating schemes, some of which are substantially different from the conventional process. Broadly speaking, the new operating schemes introduce periodic modulations of selected control parameters into the operating cycle. We shall discuss concepts such as asynchronous port switching, cyclic modulation of feed concentration, time-variable manipulation of the flow rates, and solvent-gradient operation. We shall end our presentation with a discussion of the pros and cons of using SMBs with very few columns.
The final part deals with the use of multi-column chromatography for the purification of biomolecules. In the downstream processing of valuable therapeutic proteins, achieving high purity and high yield simultaneously, is a key prerequisite for the production of safe biopharmaceuticals at low and competitive costs. On the chromatography side, the use of cost-effective, non-affinity stationary phases and gradient chromatography in combination with multicolumn continuous chromatographic processes (MCSGP) presents an option for biopurifications. Advantages over batch chromatography include the possibility of simultaneous achievement of high yield and purity, a higher throughput and lower buffer consumption. We will discuss the fundamentals of these operations and discuss academic/industrial case studies.
The workshop is designed in such a way to require only a basic understanding of chromatography as a prerequisite; no previous exposure to the SMB technology is required. It will be an in-depth primer for the newcomers to the field, and it will offer a structured presentation of the MCC fundamentals to those aiming at a better understanding of the technology. The workshop will put all participants in the position to be able to follow and profit from the scientific presentations about MCC during the conference.
Lecturers
Arvind Rajendran is an associate professor of chemical engineering at the University of Alberta (Canada). He has authored more than 50 papers in the area of separation sciences. His research interests are in large-scale adsorption and chromatography. He is on the scientific committee of important scientific meetings and on the editorial boards of Chemical Engineering and Technology and Adsorption- The journal of the International Adsorption society. He is an associate editor of the journal” Adsorption science and technology” and serves as a director of the International Adsorption society (IAS)
Jose P. Mota is full professor of chemical and biochemical engineering at the department of chemistry of University Nova de Lisboa (Portugal). He has authored over one hundred papers in the areas of separation science and transport phenomena. He has received 8 international awards, is member of the Scientific Council of Sciences and Engineering (CCCE) of the Portuguese National Science Foundation (FCT/MCTES), and member of the Board of Directors of the International Adsorption Society (IAS).
Massimo Morbidelli received his Laurea in Chemical Engineering at the Politecnico di Milano and PhD at the University of Notre Dame. He is currently Professor of Chemical Reaction Engineering at the Institute for Chemical and Bioengineering at ETH Zurich (Switzerland). His main research interest is currently in the area of integrated continuous up and downstream processes for the purification of therapeutic proteins, their PEGylation reactions and other processes of interest in the pharmaceutical industry.
Massimo Morbidelli is co-author of more than 500 papers, 11 international patents and four books. He serves as an associate editor of the ACS journal of Industrial & Engineering Chemistry Research, and is the recipient of the 2005 R.H. Wilhelm Award in Chemical Reaction Engineering of the American Institute of Chemical Engineers and of the 2014 Gerhard Damköhler-Medaille of DECHEMA.and VDI-GVC. He is a cofounder of ChromaCon Ltd., a spin-off company from his research group, which brings new chromatographic processes (MCSGP-technology) for the purification of proteins and peptides to the market.
|
Workshop 4: Process Design for Purification of Biomolecules
Abstract
Scale up of process chromatography of large biomolecules such as proteins, plasmids, virus, and virus like particles is the topic of this workshop. An introduction to the biophysical properties of these molecules will be given and consequently, which chromatographic models apply to describe the adsorption and desorption on chromatographic media. Chromatographic media most suitable for this type of molecules will be reviewed. Scale up rules will be addressed and how chromatographic processes can be designed to reach optimal productivity and selectivity. The critical process parameters will be highlighted and mathematical models will be described to explore the range of operation. The workshop will be completed with examples from industry, showing scale-up from mg to kg and highlighting operational constraints related to industrialization.
Keywords
Protein Purification, Scale Up, Modeling, Process Design
Lecturers
Alois Jungbauer received his PhD in Food Technology and Biotechnology from the University of Natural Resources and Life Sciences Vienna, Austria 1986. He serves since then as a professor at the Department of Biotechnology. He teaches Protein Technology and Downstream Processing and Biochemical Engineering. Professor Jungbauer is head of the laboratory for Protein technology and Downstream Processing. He also acts as area head and Dep. Director of Research in the Austrian Centre of Industrial Biotechnology. He is currently working in the field of bioengineering of proteins, plasmids and viruses with special focus on expression, downstream processing and characterization of large biomolecules. As a proliferate researcher he has more than 250 publications on recombinant protein production and bioseparation, 15 patents and 12 book contributions and recently a monograph entitled “Protein Chromatography, Process Development and Scale Up”. He is executive editor and co-founder of Biotechnology Journal, and member of editorial boards from numerous journals in the area of biochemical engineering.
Bernt Nilsson is professor at Department of Chemical Engineering, Lund University, Sweden. He received his PhD in Automatic Control in 1993 and has worked in the area of process engineering and process control ever since and is responsible for teaching Transport Phenomena and Advanced Process Simulation at Lund University. His research activities are in the field of mathematical modelling and simulation techniques, model calibration and optimization techniques, model based operation and control, model based engineering and computer tools with main applications in protein chromatography for biopharmaceutical applications, and industrial chromatographic systems in pharmaceutical industries. He also conducts Industrial courses in industrial process simulation and process engineering. He has more than 100 peer reviewed publications in the field of process engineering and chromatography.
|
|
Gold Sponsor
Silver Sponsors
Bronze Sponsor
Lunch Sponsor
Media Partners
|



